Publications 2016
| 24. Selective Tsuji-Trost type C-allylation of hydrazones: a straightforward entry into 4,5-dihydropyrazoles. El Mamouni, E. H.; Cattoen, M.; Cordier, M.; Cossy, J.; Arseniyadis, S.; Ilitki, H.; El Kaim, L. Chem. Commun. 2016, 52 (100), 14490-14493. http://dx.doi.org/10.1039/C6CC08171A |
|
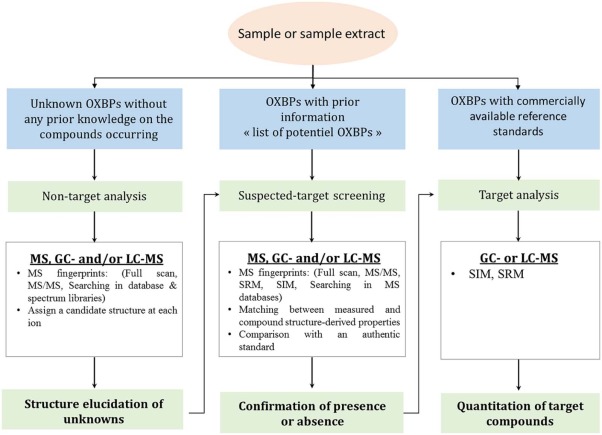 |
23. Formation and determination of organohalogen by-products in water. Part III. Characterization and quantitative approaches. Kinani, A.; Kinani, S.; Bouchonnet, S. Trends Anal. Chem. 2016, 85, 295-305. https://doi.org/10.1016/j.trac.2016.09.013 |
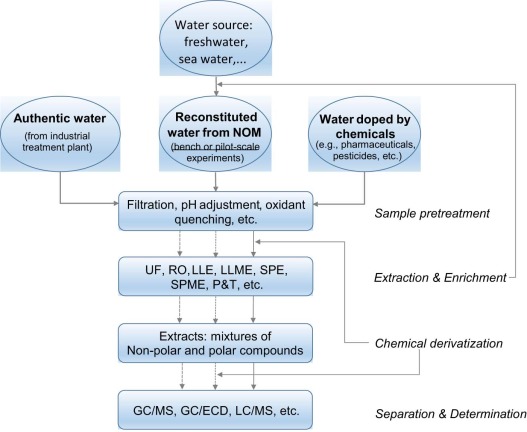 |
22. Formation and determination of organohalogen by-products in water. Part II. Sample preparation techniques for analytical approaches. Kinani, A.; Kinani, S.; Bouchonnet, S. Trends Anal. Chem. 2016, 85, 281-294. https://doi.org/10.1016/j.trac.2016.07.006 |
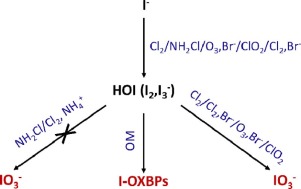 |
21. Formation and determination of organohalogen by-products in water. Part I. Discussing the parameters influencing the formation of organohalogen by-products and the relevance of estimating their concentration using the AOX (adsorbable organic halide) method. Kinani, A.; Kinani, S.; Richard, B.; Lorthioy, M.; Bouchonnet, S. Trends Anal. Chem. 2016, 85, 273-280. https://doi.org/10.1016/j.trac.2016.06.008 |
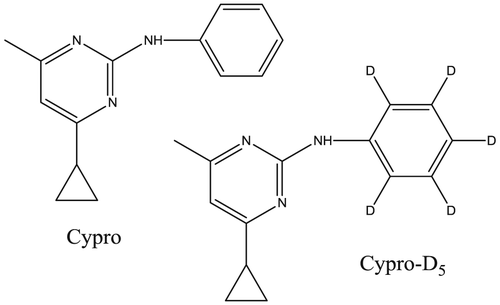 |
20. Photodegradation of cyprodinil under UV–visible irradiation – chemical and toxicological approaches. Nicol, E.; Chayata, H.; Genty, C.; Bouchonnet, S.; Bourcier, S. Rapid Commun. Mass Spectrom. 2016, 30 (19), 2201-2211. http://dx.doi.org/10.1002/rcm.7685 |
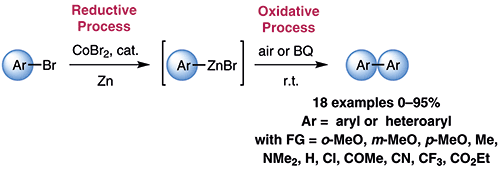 |
19. Cobalt-catalyzed oxidative homocoupling of arylzinc species. Bourne-Branchu, Y.; Moncomble, A.; Corpet, M.; Danoun, G.; Gosmini, C. Synthesis 2016, 48 (19), 3352-3356. http://dx.doi.org/10.1055/s-0035-1562488 |
| 18. Synthesis of symmetrical diaryl ketones by cobalt-catalyzed reaction of arylzinc reagents with ethyl chloroformate. Rérat, A.; Michon, C.; Agbossou-Niedercorn, F.; Gosmini, C. Eur. J. Org. Chem. 2016, 2016 (26), 4554-4560. http://dx.doi.org/10.1002/ejoc.201600738 |
|
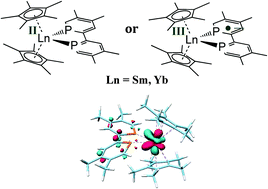 |
17. Electron transfer in tetramethylbiphosphinine complexes of Cp*2Yb and Cp*2Sm. Jaoul, A.; Clavaguera, C.; Nocton, G. New J. Chem. 2016, 40 (8), 6643-6649. http://dx.doi.org/10.1039/C6NJ00527F |
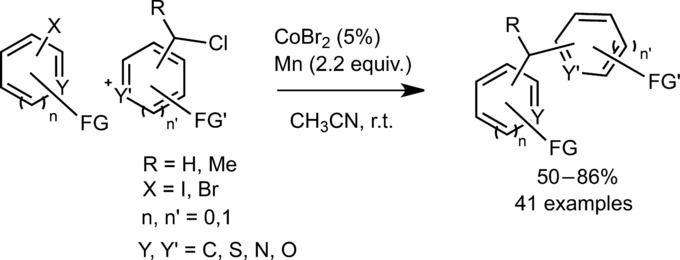 |
16. Cobalt-catalyzed reductive cross-coupling between benzyl chlorides and aryl halides. Pal, S.; Chowdhury, S.; Rozwadowski, E.; Auffrant, A.; Gosmini, C. Adv. Synth. Catal. 2016, 358 (15), 2431-2435. http://dx.doi.org/10.1002/adsc.201600378 |
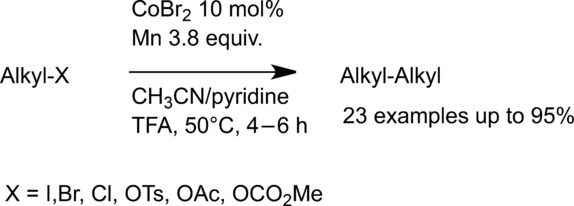 |
15. Cobalt-catalyzed Csp3 −Csp3 homocoupling. Cai, Y.; Qian, X.; Gosmini, C. Adv. Synth. Catal. 2016, 358 (15), 2427-2430. http://dx.doi.org/10.1002/adsc.201600213 |
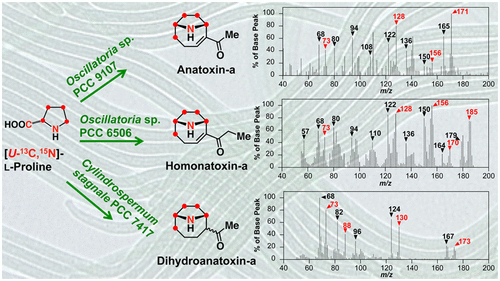 |
14. Dihydroanatoxin-a is biosynthesized from proline in cylindrospermum stagnale PCC 7417: isotopic incorporation experiments and mass spectrometry analysis. Méjean, A.; Dalle, K.; Paci, G.; Bouchonnet, S.; Mann, S.; Pichon, V.; Ploux, O. J. Nat. Prod. 2016, 79 (7), 1775-1782. http://dx.doi.org/10.1021/acs.jnatprod.6b00189 |
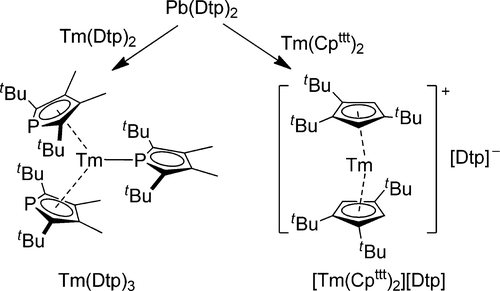 |
13. Synthesis and characterization of 1,1′-diphosphaplumbocenes: oxidative ligand transfer reactions with divalent thulium complexes. Jaroschik, F.; Momin, A.; Martinez, A.; Harakat, D.; Ricard, L.; Le Goff, X.-F.; Nocton, G. Organometallics 2016, 35 (11), 2032-2038. http://dx.doi.org/10.1021/acs.organomet.6b00313 |
| 12. Cascade metathesis reactions for the synthesis of taxane and isotaxane derivatives. Ma, C.; Letort, A.; Aouzal, R.; Wilkes, A.; Maiti, G.; Farrugia, L. J.; Ricard, L.; Prunet, J. Chem. Eur. J. 2016, 22 (20), 6891-6898. http://dx.doi.org/10.1002/chem.201600592 |
|
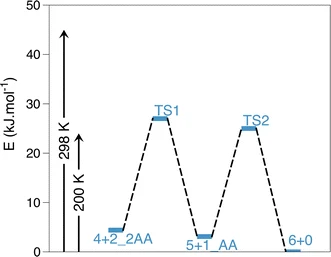 |
11. Theoretical insight into the coordination number of hydrated Zn2+ from gas phase to solution. Jana, C.; Ohanessian, G.; Clavaguéra, C. Theor. Chem. Acc. 2016, 135 (5), 141. https://doi.org/10.1007/s00214-016-1887-8 |
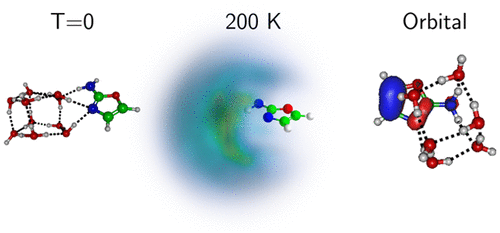 |
10. Stepwise hydration of 2-aminooxazole: theoretical insight into the structure, finite temperature behavior and proton-induced charge transfer. Calvo, F.; Bacchus-Montabonel, M. C.; Clavaguéra, C. J. Phys. Chem. A 2016, 120 (15), 2380-2389. http://dx.doi.org/10.1021/acs.jpca.5b12392 |
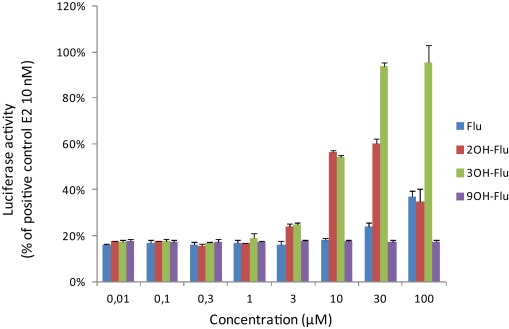 |
9. Photodegradation of fluorene in aqueous solution: Identification and biological activity testing of degradation products. Kinani, S.; Souissi, Y.; Kinani, A.; Vujović, S.; Aït-Aïssa, S.; Bouchonnet, S. J. Chromatogr. A 2016, 1442, 118-128. https://doi.org/10.1016/j.chroma.2016.03.012 |
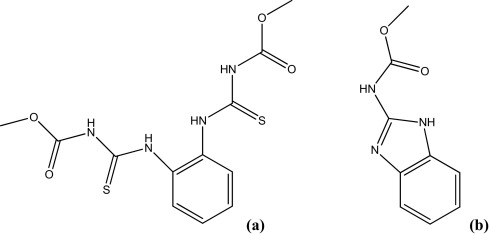 |
8. Characterization of the ultraviolet–visible photoproducts of thiophanate-methyl using high performance liquid chromatography coupled with high resolution tandem mass spectrometry—Detection in grapes and tomatoes. Chayata, H.; Lassalle, Y.; Nicol, É.; Manolikakes, S. M.; Souissi, Y.; Bourcier, S.; Gosmini, C.; Bouchonnet, S. J. Chromatogr. A 2016, 1441, 75-82. https://doi.org/10.1016/j.chroma.2016.02.078 |
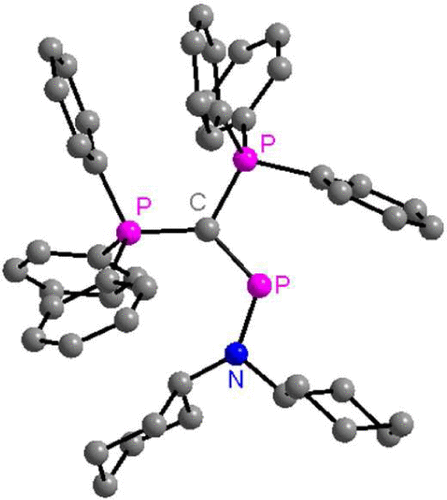 |
7. Preparation, structural analysis, and reactivity studies of phosphenium dications. Tay, M. Q. Y.; Ilić, G.; Werner-Zwanziger, U.; Lu, Y.; Ganguly, R.; Ricard, L.; Frison, G.; Carmichael, D.; Vidović, D. Organometallics 2016, 35 (4), 439-449. http://dx.doi.org/10.1021/acs.organomet.5b00763 |
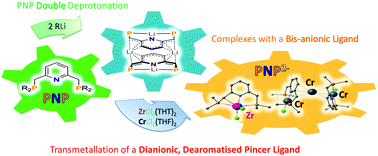 |
6. Direct synthesis of doubly deprotonated, dearomatised lutidine PNP Cr and Zr pincer complexes based on isolated K and Li ligand transfer reagents. Simler, T.; Frison, G.; Braunstein, P.; Danopoulos, A. A. Dalton Trans. 2016, 45 (7), 2800-2804. http://dx.doi.org/10.1039/C6DT00144K |
| 5. A practical cobalt-catalyzed cross-coupling of benzylic zinc reagents with aryl and heteroaryl bromides or chlorides. Benischke, A. D.; Knoll, I.; Rerat, A.; Gosmini, C.; Knochel, P. Chem. Commun. 2016, 52 (15), 3171-3174. http://dx.doi.org/10.1039/C5CC10272C |
|
| 4. Stereoselectivity of Michael addition of P(X)–H-type nucleophiles to cyclohexen-1-ylphosphine oxide: the case of base-selective transformation. Jaklińska, M.; Cordier, M.; Stankevič, M. J. Org. Chem. 2016, 81 (4), 1378-1390. http://dx.doi.org/10.1021/acs.joc.5b02337 |
|
| 3. Palladium(II) complexes featuring a mixed phosphine-pyridine-iminophosphorane pincer ligand: synthesis and reactivity. Cheisson, T.; Auffrant, A. Dalton Trans. 2016, 45 (5), 2069-2078. http://dx.doi.org/10.1039/C5DT02789F |
|
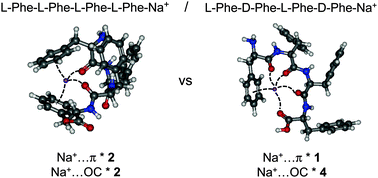 |
2. Chirality-dependent structuration of protonated or sodiated polyphenylalanines: IRMPD and ion mobility studies. Lepère, V.; Le Barbu-Debus, K.; Clavaguera, C.; Scuderi, D.; Piani, G.; Simon, A.-L.; Chirot, F.; MacAleese, L.; Dugourd, P.; Zehnacker, A. Phys. Chem. Chem. Phys. 2016, 18 (3), 1807-1817. http://dx.doi.org/10.1039/C5CP06768E |
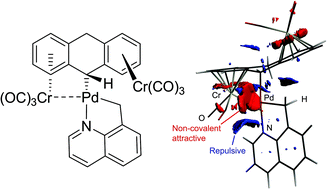 |
1. New Pd(II) hemichelates devoid of incipient bridging CO⋯Pd interactions. |


